
- #Are lone pairs sigma bonds zip file
- #Are lone pairs sigma bonds license
- #Are lone pairs sigma bonds download
This leaves one sp hybrid on each atom to either bond to hydrogen (C) or hold a lone pair of electrons (N). Because the nitrogen atom can also be described as sp hybridized, we can use one sp hybrid on each atom to form a C–N σ bond. Describe the bonding in HCN.Ī Because HCN is a linear molecule, it is likely that the bonding can be described in terms of sp hybridization at carbon. Use any remaining unhybridized p orbitals to form π and π* orbitals.Ĭ Fill the orbitals with the remaining electrons in order of increasing energy. Use the hybrid orbitals to form the σ-bonded framework of the molecule and determine the number of valence electrons that are used for σ bonding.ī Determine the number of remaining valence electrons.
Given: chemical compound and molecular geometryĪsked for: bonding description using hybrid atomic orbitals and molecular orbitalsĪ From the geometry given, predict the hybridization in HCN. The two CH 2 fragments are coplanar, which maximizes the overlap of the two singly occupied 2 p z orbitals.ĭescribe the bonding in HCN using a combination of hybrid atomic orbitals and molecular orbitals.
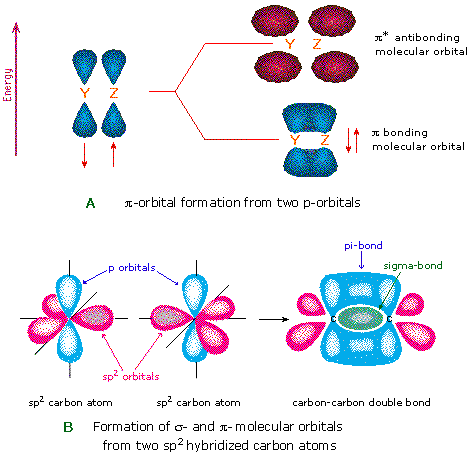
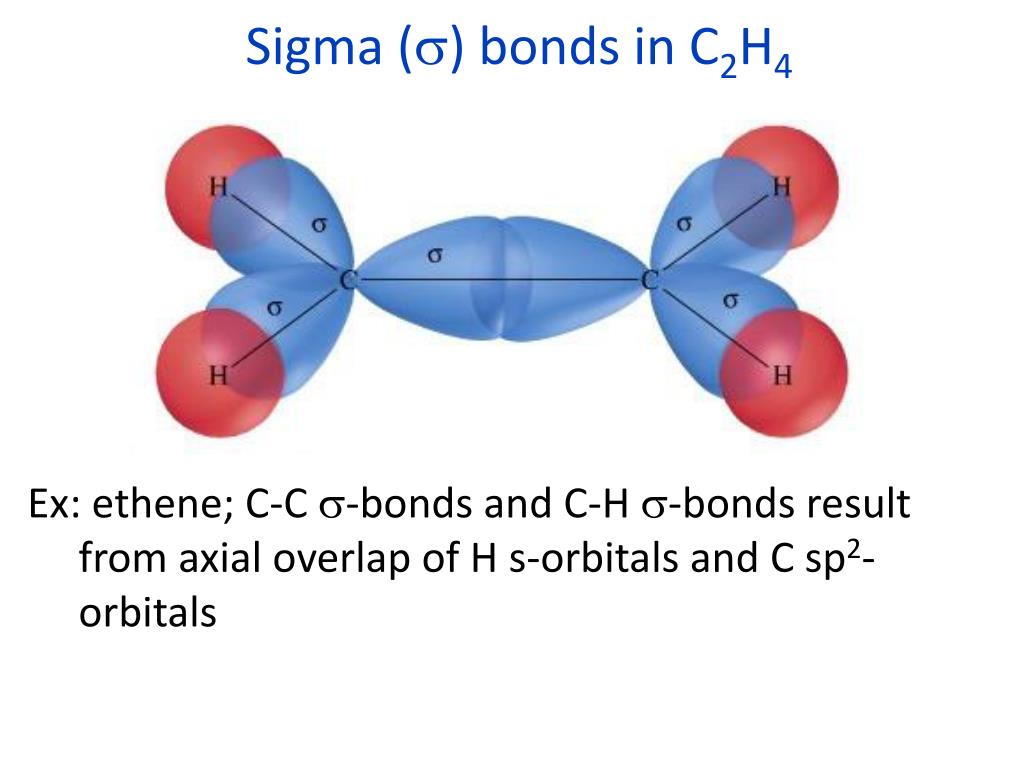
Our model is supported by the facts that the measured carbon–carbon bond is shorter than that in ethane (133.9 pm versus 153.5 pm) and the bond is stronger (728 kJ/mol versus 376 kJ/mol in ethane). Consequently, the C–C bond in ethylene consists of a σ bond and a π bond, which together give a C=C double bond. Because each 2 p z orbital has a single electron, there are only two electrons, enough to fill only the bonding (π) level, leaving the π* orbital empty. The π* orbital lies outside the internuclear region and has a nodal plane perpendicular to the internuclear axis. With the formation of a π bonding orbital, electron density increases in the plane between the carbon nuclei. The two singly occupied 2 p z orbitals can overlap to form a π bonding orbital and a π* antibonding orbital, which produces the energy-level diagram shown in Figure 9.33 "Molecular Orbital Energy-Level Diagram for π Bonding in Ethylene". (Note: by convention, in planar molecules the axis perpendicular to the molecular plane is the z-axis.)Īfter hybridization, each carbon still has one unhybridized 2 p z orbital that is perpendicular to the hybridized lobes and contains a single electron (part (b) in Figure 9.32 "Bonding in Ethylene"). (b) One singly occupied unhybridized 2 p z orbital remains on each carbon atom to form a carbon–carbon π bond. This uses 10 of the 12 valence electrons to form a total of five σ bonds (four C–H bonds and one C–C bond). (a) The σ-bonded framework is formed by the overlap of two sets of singly occupied carbon sp 2 hybrid orbitals and four singly occupied hydrogen 1 s orbitals to form electron-pair bonds.
#Are lone pairs sigma bonds zip file
zip file containing this book to use offline, simply click here.
#Are lone pairs sigma bonds download
You can browse or download additional books there. More information is available on this project's attribution page.įor more information on the source of this book, or why it is available for free, please see the project's home page. Additionally, per the publisher's request, their name has been removed in some passages. However, the publisher has asked for the customary Creative Commons attribution to the original publisher, authors, title, and book URI to be removed. Normally, the author and publisher would be credited here. This content was accessible as of December 29, 2012, and it was downloaded then by Andy Schmitz in an effort to preserve the availability of this book.
#Are lone pairs sigma bonds license
See the license for more details, but that basically means you can share this book as long as you credit the author (but see below), don't make money from it, and do make it available to everyone else under the same terms. This book is licensed under a Creative Commons by-nc-sa 3.0 license.


 0 kommentar(er)
0 kommentar(er)
